For an interesting molecule to become a drug, it needs efficacy, exposure and safety, all within a reasonable dose. My background, developed over the past two decades, is focused on optimizing the pharmacokinetic, pharmacodynamic and safety profiles of drug candidates. I have progressed over a dozen small molecule programs from lead optimization into first in human clinical trials and as a consultant to your company’s program I can assist with the following:
- Help define an exposure profile appropriate to client’s pharmacology.
- Assist chemistry teams in designing new molecules with desired physiochemical and metabolic properties.
- Work with CROs to set up appropriate testing funnels and pharmacology models
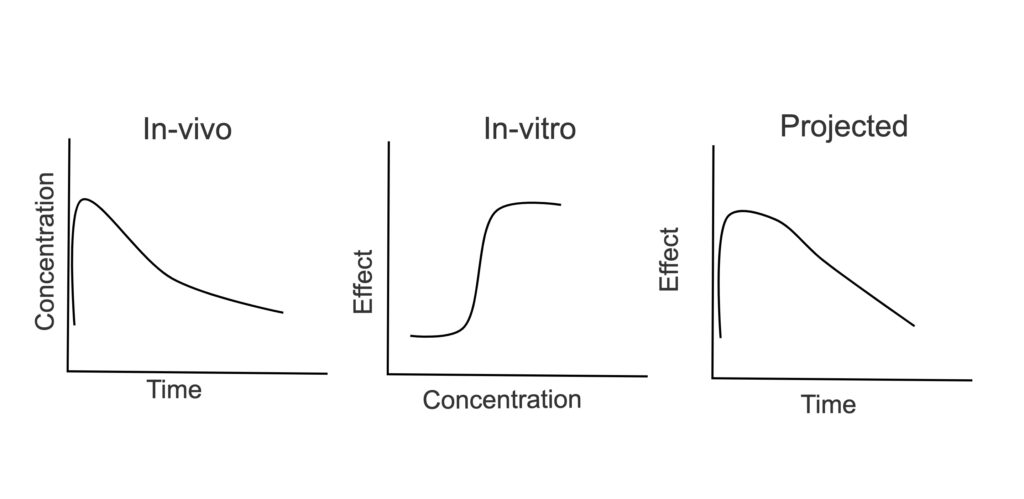
- Interpret in-vitro data to prioritize molecules for in vivo testing.
- Interpret PK, PD and TK data to support project progress.
- Design PKPD studies to determine exposure vs. effect relationships.
- Provide estimates of human dose to support all stages of discovery and development.
- Prepare and author relevant sections of your FIH submission (IND, CTN, etc) as well as provide relevant content for clinical protocols and investigator brochures.
- Provide guidance on preclinical program strategy.